SumitCreator
what is the status of the vaccine of which company / organization?
The he vaccine race to safeguard against coronaviruses has reached the goal. Four companies have announced the results of the last phase of their phase-3 trials, ie, human trials. Till now, all four have declared their effective vaccine rate of 90% or more practical. Actually, it's the affixation rate that forms the premise for approving a vaccine. Let's understand what it's and the way it's extracted ...
How is that the vaccine affixed?
It is an extended and sophisticated process. When the researcher trials a vaccine, half the people involved in it apply the vaccine and half the people give placebo.
Volunteers are then monitored for some months and through this point there are separate investigations. Blood tests see whether antibodies have developed within the body. Awaits what number people from which group are positive for the virus.
In Pfizer's case, the corporate dodged 44 thousand Volunteers. Over some months of monitoring, 170 people were found to be corona positive. of those 162 people got placebo, while 8 people were from the vaccine group.
Based on these numbers, Pfizer's researchers performed calculations. The group which failed to get the vaccine received more positive patients. Similarly, positive patients were less within the vaccine group. This led to the removal of the affixes.
According to scientists, the difference between folks that got sick after getting vaccinated and people who got sick without vaccine is affixes. If no difference was found between the 2 groups, then the Efficacy would became 0 At the identical time, if someone weren't sick from the vaccine group, then the Efficie would are 100%.
A 90% or more afficacy means the vaccine is functioning well. But this number doesn't tell you ways much you're likely to urge sick if you apply the vaccine. For this reason, it can't be said that after the introduction of the vaccine, Covid-19 are finished worldwide.
At present, 216 vaccines are currently under operation to shield against coronaviruses worldwide. in line with the WHO vaccine landscape, there are 163 vaccines in pre-clinical trials. That is, this vaccine is currently being tested in labs only. At the identical time, 53 vaccines are in clinical trials. Clinical trials typically take several years. But considering Corona as an emergency, some countries have given emergency approval to its vaccine. At present, 4 in China, 2 in Russia and 1 in UK are given emergency approval. the method of emergency approval has started in other countries furthermore. 7 vaccines are still in phase-3 trials and haven't been approved anywhere. At the identical time, 3 vaccines are in phase-2 trials and 13 are in vaccine phase-1/2 trials. Now 23 vaccines are within the first phase of human trials.
Trials of 4 vaccine results, up to 90% effective
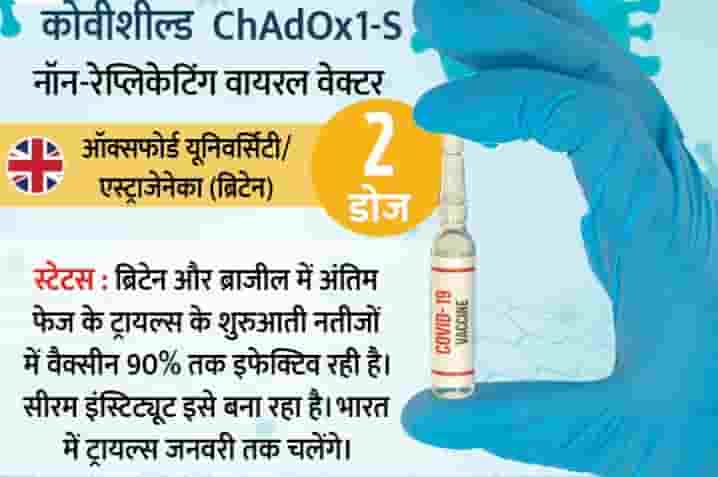
1. Oxford University / AstraZeneca
Oxford University and AstraZeneca have created the vaccine (CoveShield), which has been found to be up to 90% effective in early results. Adar Poonawala, CEO of Serum Institute of India, the world's leading vaccine production company, said that within period of time, emergency approval will apply in India.
2. Pfizer and Bioentech
American Pharma company Pfizer and German Corona vaccine joint Corona vaccine has proved to be 95% effective in Phase-3 trial. In the UK, this vaccine has been given emergency approval on 2 December. an identical approval has also been given to the US Food and Drug Administration (USFDA).
3. Moderna (America)
4. Gamalaya Research Institute (Russia)
The Russian-made vaccine has proven to be 95% effective in fighting corona during the Sputnik V trial. this is often revealed within the second preliminary analysis of the run. 28 days after the primary dose, this vaccine showed 91.4% efficacy. The Russian body has an agreement with Dr. Reddy's Laboratory and Phase-2/3 Combined Trials have started in India.
4 in China, 1 vaccine in Russia, emergency approval before trials
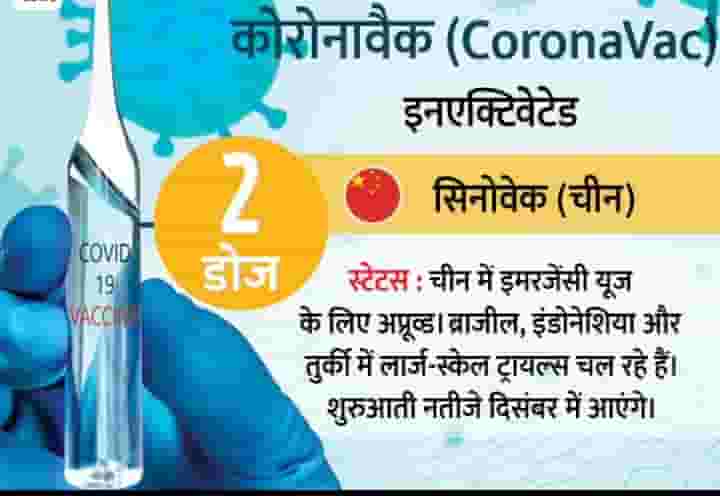
1. Sinovec (China)
The results of Phase-1/2 trials of the inactivated vaccine of Chinese private company Sinowec came come in June. the corporate then included 743 Volunteers within the trials and none of them showed any serious symptoms. The results of the trials were announced in November itself. the ultimate phase trials began in Brazil, Indonesia and Turkey. to date it's not yielded results.
2. Wuhan Candidate
Chinese government company Sinopharm, together with Wuhan Institute of Biological Products, has created this vaccine. Its phase-1/2 trials yielded good results. Phase-3 trials began in Peru, Morocco and therefore the UAE. In September the UAE gave emergency approval to the Sinofarm vaccine.
3. Beijing Candidate
The vaccine, developed by the Beijing Institute of Biological Products, is about to be launched within the Chinese state-owned Sinopharm market. it's also received emergency approval in China moreover as within the UAE.
4.Cansino Biologics
The Chinese company, Cancino Biologics, created the vaccine together with the research institute of the Chinese military. it had been approved by the Chinese military on 25 June for a year. the corporate then conducted phase-3 trials in Saudi Arabia, Pakistan and Russia, but the results haven't been announced yet.
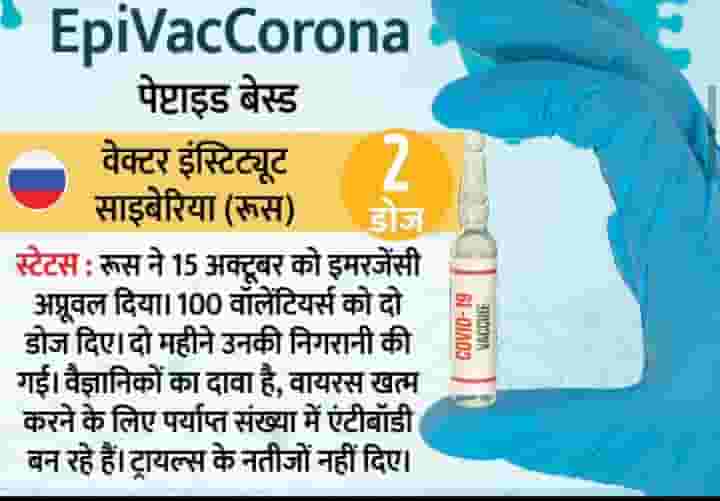
5. Vector Institute of Siberia
Russia gave emergency approval to EpiVacCorona, the coronavirus vaccine of Siberia's Vector Institute, on 15 October. within the initial stage, the vaccine was administered to 100 volunteers within the placebo-controlled human trials. Scientists claim that this vaccine has antibodies within the soma for a minimum of six months.
5 vaccines including India's indigenous vaccine in final round
1. Kovaxin of India Biotech (India)
Phase-3 trials of indigenous vaccine - covaxin, which are considered special for India, have started. These trials are going down at around 25 sites. This vaccine has been developed by Hyderabad-based company Bharat Biotech unitedly with the National Institute of Virology (NIV) and therefore the Indian Council of Medical Research (ICMR). The vaccine has been effective within the trials till date, now the results of phase-3 trials are awaited.
2. Janssen Pharma (US)
The inactivated vaccine by Janssen Pharma, a subsidiary of yankee company Johnson & Johnson, has proved effective in Phase-1/2 trials. Its final phase trials are happening at 60 thousand people.
3. Novavax (America)
The vaccine of the US-based company Novavax is additionally eagerly awaited within the US. the corporate has engaged with Vaccine Manufacturing at Serum Institute of India, Pune.
4. Medicargo (Canada)
Canadian drug developer Medicargo has also launched Phase-2/3 Combined Trials. within the initial stage, the company's vaccine and vaccine boosters of GlaxoSmithKline (GSK) are successful in making antibodies that eliminate the virus. Medicargo has the support of Mitsubishi Tanabe Pharma and Tobacco giant Philip Morris. 5. Anhui Zhifei Longcom Biopharmaceutical Group (China)
5. Anhui Zhifei Longcom Biopharmaceutical Group (China)
The vaccine trials developed by a unit of Chongqing Zifai Biological Products started in June. This vaccine has been created by Anhui Zhifai Longcom Biopharmaceuticals and Chinese Academy of Science
.Trials of 16 companies within the second phase, but 3 of our work
1. Cadila Healthcare (India)
At present, trials of 16 companies are within the second phase. But among them, 3 companies including Ahmedabad-based Cadila Healthcare are within the second phase. The trials of Phase-1 and Phase-2 of the vaccine of the remaining 13 companies are occurring simultaneously. Cadila Healthcare vaccine results are revealed soon. it's expected that this vaccine also will be available by April-2021.
2. Xiamen University (China)
The Chinese University of China has created a nasal vaccine unitedly with metropolis University and a Beijing-based company. Its Phase-1 results are successful. it's currently in Phase-2 trials. it'll be sort of a nasal spray and rather than injecting, just spraying within the nose are enough.
3. Curevac (Germany)
Trials of the vaccine from German company Curevac have also gained momentum within the recent past. The vaccine has proved effective to this point. due to this, the corporate has established a network to deliver its vaccine to all or any countries of Europe.

























1 comment:
I want the vaccine so badly. I can't wait for it to come.
Post a Comment